Synthesis of three-dimensionally ordered macroporous composite Ag/Bi2O3–TiO2 by dual templates and its photocatalytic activities for degradation of organic pollutants under multiple modes†
Abstract
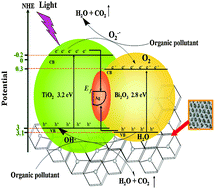
In this study, polystyrene latex spheres and triblock copolymer surfactant EO20PO70EO20 were used simultaneously as dual templates, with TiO2 as a substrate to fabricate the three-dimensionally ordered macroporous (3DOM) composite Ag/Bi2O3–TiO2 by a sol–gel method and post-processing calcination. The phase structure, chemical composition, morphology, and surface physicochemical properties of as-prepared samples were well characterized by X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), Ultraviolet-visible diffuse reflectance (UV-Vis/DRS), X-ray photoelectron spectroscopy (XPS), Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), and Nitrogen adsorption–desorption measurements. The investigations revealed a distinct crystalline phase structure of the composite with a uniform and ordered spherical cavity array of periodic arrangement, attributed to highly three-dimensionally ordered macroporous structure with mesoporous walls. Compared to TiO2, the light absorption of 3DOM Ag/Bi2O3–TiO2 was red-shifted, ca. 100 nm, to the visible region. The effectiveness of 3DOM Ag/Bi2O3–TiO2 mediated methods for photocatalytic degradation of crystal violet by employing multi-modes such as UV, simulated solar light, and microwave-assisted irradiation was significantly enhanced compared to that of P25, Bi2O3, and Ag/Bi2O3–TiO2. Moreover, the 3DOM Ag/Bi2O3–TiO2 composite displayed excellent photocatalytic activity toward degradation of different organic pollutants using UV light irradiation. TOC analysis of the water solution phase of centrifuged samples indicated that water solution products formed and that with continued ultraviolet radiation, the intermediates eventually mineralized, volatilized, or were converted to other products. Importantly, the photocatalytic activity of 3DOM Ag/Bi2O3–TiO2 was retained even after three cycles. Furthermore, based on the experimental results, the possible photocatalytic reaction mechanism of the as-prepared material was proposed.

 Please wait while we load your content...
Please wait while we load your content...