Influence of linker groups on the solubility of triazine dendrimers†
Abstract
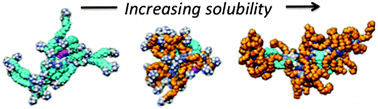
Eight triazine dendrimers were prepared to probe the impact of linker choice on water solubility. Three different linkers were assessed including two hydrophobic diamines that show high reactivity, piperazine and trismethylene bispiperidine, as well as a hydrophilic diamine, 4,7,10-trioxotridecane-1,14-diamine, which is less reactive. Dendrimers 1–8 share a common, generation two, hydrophobic core, 1. Dendrimer 1 is insoluble in water. Of the three generation four dendrimers, 2–4, that were prepared, 2 is also insoluble in water, but substitution of one or two of the hydrophobic linkers with 4,7,10-trioxotridecane-1,14-diamine yields sparingly soluble 3 and more soluble 4, respectively. Molecular dynamics simulations of dendrimers 2–4 in water provide additional insight into their shape, hydration and hydrophobicity. Generation six targets, 5–8, are also sensitive to choice of interior and surface groups. Dendrimer 5 is insoluble in water, but replacing one or two hydrophobic linkers with 4,7,10-trioxotridecane-1,14-diamine yields dendrimers 6 and 7 with modest affect unless the double substitution occurs in tandem at the periphery to yield 8 which shows high solubility in water. The solubility trends suggest that the choice of cationic surface group is critical, and that piperazine groups on the periphery and interior do little to promote solubility of triazine dendrimers in water compared with the hydrophilic amine 4,7,10-trioxotridecane-1,14-diamine.

 Please wait while we load your content...
Please wait while we load your content...