Exploring the reaction mechanism of a cationic terminal iridium methylene complex with ethyl diazoacetate, a Lewis base and dihydrogen: a quantum chemistry study†
Abstract
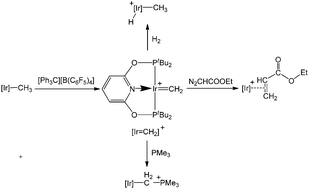
The mechanisms for the formation of a cationic methylene complex [(PONOP)Ir(CH2)]+ (PONOP = 2,6-bis(di-tert-butylphosphinito)pyridine) via α-hydride abstraction from a neutral methyl complex [(PONOP)Ir(CH3)] and its reactivity with ethyl diazoacetate, a Lewis base (PMe3) and dihydrogen have been studied computationally with the aid of density functional theory (DFT). The calculation results show that the η2-alkene complex can be formed via a direct C–C coupling reaction involving the methylene ligand and ethyl diazoacetate. A ylide compound is given for the reaction of this cationic methylene complex with PMe3. In addition, hydrogenolysis of the Ir![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 moiety of this cationic methylene complex results in a hydride iridium complex. Our calculations could provide new insights into the reactivity of methylene complexes.
CH2 moiety of this cationic methylene complex results in a hydride iridium complex. Our calculations could provide new insights into the reactivity of methylene complexes.

 Please wait while we load your content...
Please wait while we load your content...