Facile synthesis and morphogenesis of superparamagnetic iron oxide nanoparticles for high-performance supercapacitor applications
Abstract
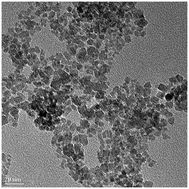
A facile method has been developed for the synthesis of nearly mono-dispersed iron oxide nanocrystals. The structural analysis of the synthesized iron oxide nanocrystals reveals the magnetite phase of Fe3O4. The average particle size of the iron oxide was estimated to be 8 ± 2 nm. The observed particle size is in good correlation with the particle size estimated by magnetic measurements. Furthermore, these nanocrystals showed bi-functional ferromagnetic and superparamagnetic behavior below and above the blocking temperature, respectively. The potential use of these nanocrystals as an electrode for supercapacitors was examined by investigating the electrochemical behavior of iron oxide using cyclic voltammetry (CV) and galvanostatic charge–discharge tests. The CV characteristics of the iron oxide electrode showed a typical pseudocapacitive behavior in 3 M KOH solution. Moreover, the specific capacitance of 185 F g−1 at the current of 1 mA was observed with excellent cyclic stability, which is much higher than the reported value for iron oxide. The higher specific capacitance is due to the uniform nano-size of iron oxide. This work provides an ultimate facile method to synthesize nanostructured iron oxide for application in next generation energy storage materials.

 Please wait while we load your content...
Please wait while we load your content...