Tri- and tetrafluoropropionamides derived from chiral secondary amines – synthesis and the conformational studies†
Abstract
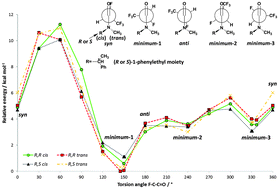
A convenient procedure for the preparation of tri- and tetrafluoropropionamides derived from R-(+)/S-(−)-N-methyl-1-phenylethylamine and cyclic pyrrolidine derivatives has been described. The X-ray analysis and the theoretical calculations have been used to study conformational analysis of obtained compounds. In contrast to single α-fluorine substituted amides, which preferred anti conformation around the F–C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, for tetrafluorinated amides the additional trifluoromethyl group forces the conformation of the F–C–C
O bond, for tetrafluorinated amides the additional trifluoromethyl group forces the conformation of the F–C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond as nearly syn.
O bond as nearly syn.

 Please wait while we load your content...
Please wait while we load your content...