Ring-opening reactions of epoxidized SWCNT with nucleophilic agents: a convenient way for sidewall functionalization†
Abstract
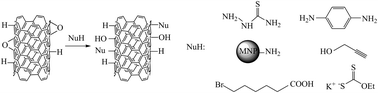
The rational chemical modification of carbon nanotubes (CNT) is crucial for their successful application in various areas of science and industry. In our approach, Birch reduction of single-walled carbon nanotubes (SWCNT) was followed by epoxidation with dimethyldioxirane (DMDO). Next, ring-opening reactions with thiosemicarbazide, p-phenylenediamine, aminosilica-coated magnetic nanoparticles, propargyl alcohol, 6-bromohexanoic acid, and ethyl potassium dithiocarbonate were performed, which resulted in a wide variety of functional groups being covalently anchored to the sidewalls of the nanotubes. Raman spectroscopy, Fourier transform infrared spectroscopy (FT-IR), ultraviolet-visible-near infrared spectroscopy (UV-Vis-NIR), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), and transmission electron microscopy (TEM) were employed to confirm modifications and study the chemical composition of the obtained structures.

 Please wait while we load your content...
Please wait while we load your content...