Eco-friendly synthesis of bimetallic AuAg nanoparticles
Abstract
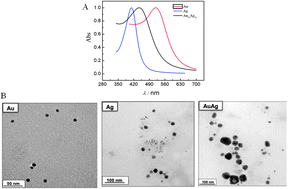
In this study we report a friendly route to produce colloidal AuAg bimetallic nanoparticles (NPs) using glycerol as a reducing chemical in alkaline medium. Ultraviolet spectroscopy (UV-Vis) and transmission electron microscopy (TEM) confirmed the formation of colloidal NPs. The nanoparticles were also directly produced on carbon to yield Au/C, Ag/C and AuAg/C. Extended X-ray absorption fine-structure (EXAFS) spectroscopy at the Au L3 edge evidenced surface segregation of silver in the AuAg/C material. Also the X-ray absorption near edge structure (XANES) region indicated a slight reduction in the Au 5d band occupancy due to the presence of silver. Since a fuel cell is a potent source of energy for the future, the catalytic properties of the NPs were investigated for anodic glycerol electro-oxidation reaction and results for AuAg NPs are compared with those for monometallic Au and Ag NPs. When compared with Au/C, the AuAg/C material displayed lower potential for glycerol electro-oxidation, reducing by 120 mV the onset potential for glycerol electro-oxidation. This means that the electro-oxidation of glycerol on the bimetallic catalyst is mainly affected by the ligand effect.

 Please wait while we load your content...
Please wait while we load your content...