Regioselective β-pyrrolic electrophilic substitution of hydrodipyrrin–dialkylboron complexes facilitates access to synthetic models for chlorophyll f†
Abstract
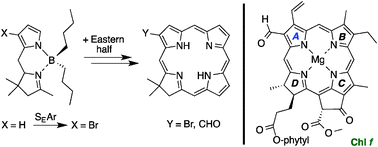
Substituents in ring A of chlorophylls can exert profound effects on spectral properties. A de novo route to synthetic chlorins employs a tetrahydrodipyrrin reactant containing pyrrole and pyrroline rings. Complexation of the tetrahydrodipyrrin with a dialkylboron motif caused electrophilic substitution (bromination, formylation) to proceed predominantly at the β7- rather than α-position of the pyrrole ring, whereas an analogous dihydrodipyrrin underwent substitution equally at the 7- and 8-positions. The fully unsaturated dipyrrin–difluoroboron complex is known to undergo electrophilic substitution at the 8-position. The 7-position of the hydrodipyrrin ultimately gives rise to substituents at the chlorin 2-position (ring A), which heretofore has been little accessed. The position of substitution was confirmed by four single-crystal X-ray structures. Two isomeric formylchlorins were prepared by Pd-mediated carbonylation of the corresponding bromochlorins. Access to a 2-formylchlorin relied on bromination of the tetrahydrodipyrrin–dibutylboron complex, whereas a 3-formylchlorin was prepared by installation of the bromo group in the earliest precursor, pyrrole-2-carboxaldehyde. The two formylchlorins differ in absorption spectral properties: the Qy absorption maximum is 654 or 664 nm for the 2- or 3-formylchlorin, respectively. The synthetic formylchlorins provide initial models for understanding the strong red absorption of native 2- or 3-formylchlorophylls (f and d).

 Please wait while we load your content...
Please wait while we load your content...