Stereoselective synthesis of Z-acrylonitrile derivatives: catalytic and acetylcholinesterase inhibition studies†
Abstract
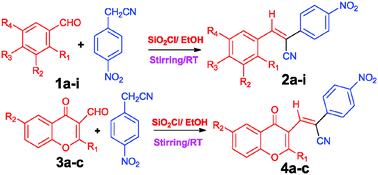
In the present study, a focused library of (Z)-acrylonitrile analogues (library A & B) were synthesized, which were typically accessed via a facile Knoevenagel condensation between p-nitrophenylacetonitrile and appropriately substituted aromatic aldehydes (1a–i) and 3-formyl chromones (3a–c). This new synthetic eco-friendly approach resulted in a remarkable improvement in the synthetic efficiency (83–92% yield), high purity, minimizing the production of chemical wastes without using highly toxic reagents for the synthesis and, more notably, it improved the selectivity for (Z)-acrylonitrile derivatives. By performing DFT calculations, it was found that the (Z)-isomer of compound 2b is stabilized by 2.61 kcal mol−1 more than the (E)-isomer. All of the compounds were tested for acetylcholinesterase (AChE) inhibition. Compounds 2a and 4c, displayed the strongest inhibition, with IC50 values of 0.20 μM and 0.22 μM respectively. The methoxy group at the para-position of phenyl ring A was found to be essential for AChE inhibition.

 Please wait while we load your content...
Please wait while we load your content...