Synthesis and optoelectronic properties of phenylenevinylenequinoline macromolecules†
Abstract
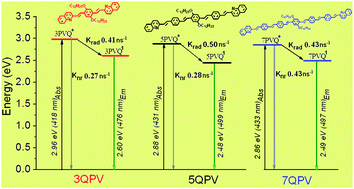
A series of 3, 5 and 7 quinoline terminated arylenevinylene oligomers was selectively synthesized by applying two repetitive reactions at each cycle of oligomerization: a Wittig and Pd Heck cross-coupling. Oligomers were characterized by 1H and homo-J-resolved, DEPT-135, APT, 13C NMR and MALDI-TOF spectrometry. A detailed characterization of the oligomers was performed by UV-Vis, static-dynamic fluorescence and Z-scan spectroscopy. The HOMO and LUMO levels were determined by cyclic voltammetry and compared with the theoretical ones. Effective conjugation is attained for the pentamer, whilst the molecular structure of the heptamer shows a pronounced torsion of the phenylenevinylene segment disrupting the degree of conjugation as revealed by theoretical simulation of the oligomer geometry. Furthermore, the theoretical simulation shows that the HOMO–LUMO frontier orbitals are mainly localized in the phenylenevinylene structure, while in the quinolines the spatial distribution is only located at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group without any appreciable contribution of the adjacent phenyl group. The pentamer is the most fluorescent oligomer with a quantum yield in solution of ϕ = 0.62–0.68 depending on the solvent and a fluorescence lifetime of 1.07–1.28 ns, making this oligomer suitable for optoelectronic devices.
N– group without any appreciable contribution of the adjacent phenyl group. The pentamer is the most fluorescent oligomer with a quantum yield in solution of ϕ = 0.62–0.68 depending on the solvent and a fluorescence lifetime of 1.07–1.28 ns, making this oligomer suitable for optoelectronic devices.

 Please wait while we load your content...
Please wait while we load your content...