Click synthesis of symmetric bis-triazol ligands and full characterisation of their copper(ii)-complexes†
Abstract
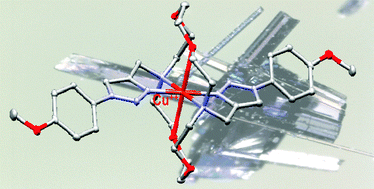
Eight novel ligands were prepared from a known symmetric diaza-18-crown-6 (cyclic ligand) and two commercial N,N′-dimethyl-alkyl diamines (acyclic ligands) via the Cu(I)-catalyzed Huisgen dipolar cycloaddition. All C2-symmetric isolated ligands readily formed stable crystalline 1 : 1-copper(II) complexes with cupric perchlorate. Their structural, electrochemical and physico-chemical properties were fully investigated with the help of X-ray diffraction, cyclic voltammetry, FT-IR, UV-visible, and electron paramagnetic resonance (EPR) spectroscopies. Planar – or nearly planar – arrangement of the two N3-triazole nitrogens and the two tertiary amine pivot nitrogens was found in one single four-coordinated species, in four five-coordinated species, and three six-coordinated species, with one or two solvent molecule(s), or two oxygen atoms of the crown ether, occupying the axial position(s) in the solid sate. The electron-donating or electron-withdrawing effect of the N1-substituent on the triazol was found to influence the Cu(II)/Cu(I) redox potential of all studied complexes in DMF. The EPR-spectrum of cyclic complexes in frozen DMF at 100 K exhibited two mononuclear species, one of them likely promoting the formation of dinuclear species as a minor component, whereas most acyclic complex spectra were quite similar.

 Please wait while we load your content...
Please wait while we load your content...