The dynamics of zinc sites in proteins: electronic basis for coordination sphere expansion at structural sites†
Abstract
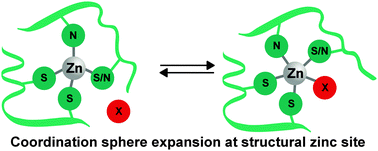
The functional role assumed by zinc in proteins is closely tied to the variable dynamics around its coordination sphere arising by virtue of its flexibility in bonding. Modern experimental and computational methods allow the detection and study of previously unknown features of bonding between zinc and its ligands in protein environment. These discoveries are occurring just in time as novel biological functions of zinc, which involve rather unconventional coordination trends, are emerging. In this sense coordination sphere expansion of structural zinc sites, as observed in our previous experiments, is a novel phenomenon. Here we explore the electronic and structural requirements by simulating this phenomenon in structural zinc sites using DFT computations. For this purpose, we have chosen MPW1PW91 and a mixed basis set combination as the DFT method through benchmarking, because it accurately reproduces structural parameters of experimentally characterized zinc compounds. Using appropriate models, we show that the greater ionic character of zinc coordination would allow for coordination sphere expansion if the steric and electrostatic repulsions of the ligands are attenuated properly. Importantly, through the study of electronic and structural aspects of the models used, we arrive at a comprehensive bonding model, explaining the factors that influence coordination of zinc in proteins. The proposed model along with the existing knowledge would enhance our ability to predict zinc binding sites in proteins, which is today of growing importance given the predicted enormity of the zinc proteome.
- This article is part of the themed collection: Zinc in the Biosciences

 Please wait while we load your content...
Please wait while we load your content...