Affinity of zinc and copper ions for insulin monomers†
Abstract
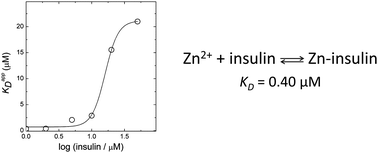
Zinc is an essential trace element involved in the correct packing and storage of insulin. Total zinc content in the pancreatic β-cells is among the highest in the body and changes in the Zn2+ levels have been found to be associated with diabetes. The most common form of the Zn–insulin complex is a hexamer containing two zinc ions. However, zinc can also form other complexes with insulin, whereas dissociation constants of these complexes are not known. We have determined that the dissociation constant value of the monomeric 1 : 1 Zn–insulin complex is equal to 0.40 μM. The apparent binding affinity decreases drastically at higher insulin concentrations where the peptide forms dimers. Cu2+ ions also bind to monomeric insulin, whereas the apparent Cu2+-binding affinity depends on HEPES concentration. The conditional dissociation constant of the Cu2+–insulin complex is equal to 0.025 μM. The analysis demonstrates that insulin cannot form complexes with zinc ions in circulation due to the low concentration of free Zn2+ in this environment.
- This article is part of the themed collection: Zinc in the Biosciences

 Please wait while we load your content...
Please wait while we load your content...