A comprehensive biological insight of trinuclear copper(ii)–tin(iv) chemotherapeutic anticancer drug entity: in vitro cytotoxicity and in vivo systemic toxicity studies
Abstract
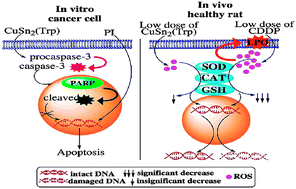
Cisplatin (cis-diamminedichloroplatinum(II), CDDP) causes severe systemic toxicity, which limits its application in cancer treatment. Nevertheless, incorporation of endogenously present essential metal ions (copper) in anticancer drug regimes in a heterometallic ligand scaffold can substantially modulate the toxic effects of non-essential metals (platinum), thereby reducing unwanted toxic side effects. A chiral L-tryptophan derived [bis(1,2-diaminobenzene) copper(II)] chloride complex [CuSn2(Trp)] was previously synthesized by us as an active chemotherapeutic agent. Furthermore, we have explored CuSn2(Trp) induced in vitro cytotoxicity in a panel of human cancer cell lines and in vivo acute and systemic toxicities in healthy female Rattus norvegicus (Wistar) rats. MTT assay showed that CuSn2(Trp) exhibits strong anticancer potency against ovarian (PA-1) and prostate carcinomas (PC-3) but lower potency towards liver (HepG2) and breast carcinomas (MCF-7). Further, flow cytometric analysis demonstrated that CuSn2(Trp) kills PA-1 cells dose-dependently after 48 h treatment. Fluorescence microscopy and western blotting revealed that the plausible mechanism behind CuSn2(Trp) cytotoxicity was apoptosis, which was substantiated by cleavage of caspase-3 and poly-(ADP-ribose) polymerase (PARP). Furthermore, it has lower toxicity than CDDP in rats as evident from its eight fold (98.11 mg kg−1) more medial lethal dose (LD50) than CDDP (12 mg kg−1). Besides, the safety profile of CuSn2(Trp) was also established and no measurable DNA damage, nephrotoxicity, hepatotoxicity and neurotoxicity were observed when assessed as a function of oxidative stress markers in contrast to CDDP at equivalent lower doses. Our findings are of high importance in the context of further in vivo cancer studies on the CuSn2(Trp) drug entity.

 Please wait while we load your content...
Please wait while we load your content...