Continuous flow photochemistry as an enabling synthetic technology: synthesis of substituted-6(5H)-phenanthridinones for use as poly(ADP-ribose) polymerase inhibitors†‡
Abstract
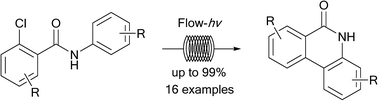
Methods utilizing continuous flow photochemistry, an enabling synthetic technology, have been developed for the generation of phenanthridinones via an intramolecular photochemical cyclization of 2-chlorobenzamides for the purposes of generating poly(ADP-ribose) polymerase inhibitors. Herein we report 16 examples of a single-step flow photocyclization which produces substituted phenanthridinones in yields up to 99%, while a two-step method leads directly to phenanthridinones from 2-chlorobenzoyl chlorides and anilines via a novel continuous flow amidation/photocyclization protocol. Overall, the flow photocyclization reactions typically progress in good to excellent yields, and in a superior fashion to analogous batch methods, greatly enabling the drug discovery process.

 Please wait while we load your content...
Please wait while we load your content...