Abstract
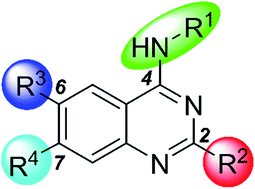
SETD8 (also known as SET8, PR-SET7, or KMT5A (lysine methyltransferase 5A)) is the only known lysine methyltransferase that catalyzes the monomethylation of histone H4 lysine 20 (H4K20). In addition to H4K20, SETD8 monomethylates non-histone substrates such as the tumor suppressor p53 and the proliferating cell nuclear antigen (PCNA). Because of its role in regulating diverse biological processes, SETD8 has been pursued as a potential therapeutic target. We recently reported the first substrate-competitive SETD8 inhibitor, UNC0379 (1), which is selective for SETD8 over 15 other methyltransferases. We characterized this inhibitor in a battery of biochemical and biophysical assays. Here we describe our comprehensive structure–activity relationship (SAR) studies of this chemical series. In addition to 2- and 4-substituents, we extensively explored 6- and 7-substituents of the quinazoline scaffold. These SAR studies led to the discovery of several new compounds, which displayed similar potencies as compound 1 and interesting SAR trends.

 Please wait while we load your content...
Please wait while we load your content...