Optimisation of a triazolopyridine based histone demethylase inhibitor yields a potent and selective KDM2A (FBXL11) inhibitor†
Abstract
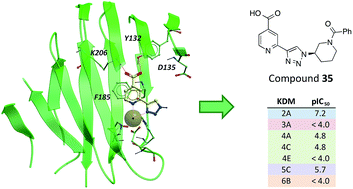
A potent inhibitor of the JmjC histone lysine demethylase KDM2A (compound 35, pIC50 7.2) with excellent selectivity over representatives from other KDM subfamilies has been developed; the discovery that a triazolopyridine compound binds to the active site of JmjC KDMs was followed by optimisation of the triazole substituent for KDM2A inhibition and selectivity.

 Please wait while we load your content...
Please wait while we load your content...