Design and chemoproteomic functional characterization of a chemical probe targeted to bromodomains of BET family proteins†
Abstract
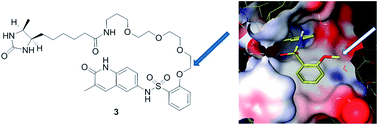
Bromodomain-containing proteins form the signal-reading element of a principal system for the control of gene expression in eukaryotes. Their potential as targets for selective drug action is increasingly being assessed and exploited. Deep characterization of the specificity, potency and other attributes of prototypical agents is an essential element of this process. Continuing studies of a dihydroquinazolinone-based series (prototype: PFI-1) with specificity for members of the BET (bromodomain and extra terminal) family led to the discovery of quinolin-2(1H)-one inhibitors with similar potency and selectivity, but increased chemical stability. Structure-guided design then led to the elaboration of a desthiobiotinylated analog retaining a high fraction of the potency of its parent compound and therefore suitable for chemoproteomic affinity capture experiments. These experiments, conducted using nuclear extracts of THP-1 cells, extended confidence in the selectivity of the series as first proposed. An additional and subsequent evaluation of specificity performed with a panel of recombinant bromodomains (BROMOscan™, DiscoveRx) supported the BET family specificity of the dihydroquinazolinone and quinolin-2(1H)-one series while adding appreciation of weaker effects shown at other bromodomains.

 Please wait while we load your content...
Please wait while we load your content...