Structure-based approaches towards identification of fragments for the low-druggability ATAD2 bromodomain†
Abstract
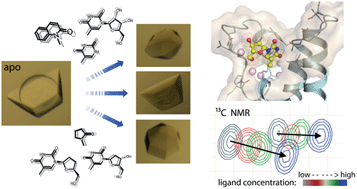
The transcriptional co-regulator ATAD2 is a prognostic marker for patient survival in many cancers. ATAD2 harbours a bromodomain which may offer an opportunity for pharmacological intervention, but its shallow, polar binding surface makes the development of inhibitors challenging. Here we optimized crystal transfer/soaking conditions enabling crystallographic fragment screening. We describe nine crystal structures of fragments including thymidine, a novel acetyl-lysine mimetic ligand and the evaluation of the binding properties of the identified fragments using NMR chemical shift perturbation experiments. The presented binding modes offer chemical starting points for the development of more potent ATAD2 inhibitors.

 Please wait while we load your content...
Please wait while we load your content...