A practical glycosyltransferase assay for the identification of new inhibitor chemotypes†
Abstract
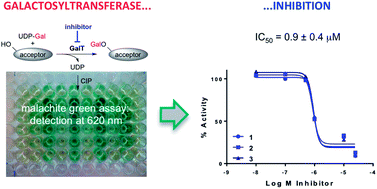
We have developed an operationally simple assay protocol for the identification and evaluation of small molecular glycosyltransferase inhibitors. Wu and co-workers have recently reported that the formation of the nucleoside diphosphate during the glycosyltransferase reaction can be monitored with a phosphatase/malachite green detection system at 620 nm. Here we demonstrate, for the first time, that this assay principle can be exploited for enzyme inhibition studies, and report the optimisation of several key parameters of this assay. We have significantly improved the reproducibility of the assay by addition of chicken egg-white lysozyme as a carrier protein. We have also substantially reduced the assay running costs by using the inexpensive and widely available calf intestinal phosphatase. Critically for inhibition studies with small organic molecules, our assay protocol tolerates additives such as the solubiliser DMSO and the surfactant Triton-X 100, and we have validated its application in inhibition experiments with two known galactosyltransferase ligands. The new assay protocol is robust and inexpensive, requires only short incubation times, and can be carried out in a microplate format. These characteristics make it ideal for high-throughput screening (HTS) campaigns. As it does not require specialist equipment and can readily be performed in non-biochemistry laboratories, we anticipate that the assay will help expedite the identification of new glycosyltransferase inhibitors both in industry and academia.

 Please wait while we load your content...
Please wait while we load your content...