A single amino acid substitution affects the substrate specificity of the seryl-tRNA synthetase homologue†
Abstract
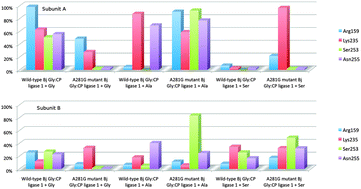
Recently described and characterized Bradyrhizobium japonicum glycine:[carrier protein] ligase 1 (Bj Gly:CP ligase 1), a homologue of methanogenic type seryl-tRNA synthetase (SerRS) is an intriguing enzyme whose physiological role is not yet known. While aminoacyl-tRNA synthetases supply ribosome with amino acids for protein biosynthesis, this homologue transfers the activated amino acid to a specific carrier protein. Despite remarkable structural similarity between the Bj Gly:CP ligase 1 and the catalytic core domain of methanogenic type SerRS, the ligase displays altered and relaxed substrate specificity. In contrast to methanogenic SerRS which exclusively activates serine, the Bj Gly:CP ligase 1 predominantly activates glycine. Besides, it shows low activity in the presence of alanine, but it is incapable of activating serine. The detailed computational study aiming to address this unexpected substrate specificity toward the small aliphatic amino acids revealed the A281G Bj Gly:CP ligase 1 mutant as the most promising candidate with reconstituted catalytic activity toward the larger substrates. The A281G mutation is predicted to increase the active site volume, allowing alanine and serine to establish important hydrogen bonds within the active site, and to adopt an optimal orientation for the reaction. The results were tested by the site-directed mutagenesis experiments coupled with in vitro kinetic assays. It was found that the A281G substitution greatly affects the enzyme specificity and allows efficient activation of both polar and small aliphatic amino acids (serine, glycine and alanine), confirming predictions and conclusions based on molecular dynamics simulations.

 Please wait while we load your content...
Please wait while we load your content...