Grafting synthetic transmembrane units to the engineered low-toxicity α-hemolysin to restore its hemolytic activity†
Abstract
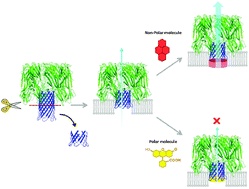
The chemical modification of proteins to provide desirable functions and/or structures broadens their possibilities for use in various applications. Usually, proteins can acquire new functions and characteristics, in addition to their original ones, via the introduction of synthetic functional moieties. Here, we adopted a more radical approach to protein modification, i.e., the replacement of a functional domain of proteins with alternative chemical compounds to build “cyborg proteins.” As a proof of concept model, we chose staphylococcal α-hemolysin (Hla), which is a well-studied, pore-forming toxin. The hemolytic activity of Hla mutants was dramatically decreased by truncation of the stem domain, which forms a β-barrel pore in the membrane. However, the impaired hemolytic activity was significantly restored by attaching a pyrenyl-maleimide unit to the cysteine residue that was introduced in the remaining stem domain. In contrast, negatively charged fluorescein-maleimide completely abolished the remaining activity of the mutants.

 Please wait while we load your content...
Please wait while we load your content...