Extracellular matrix density promotes EMT by weakening cell–cell adhesions†
Abstract
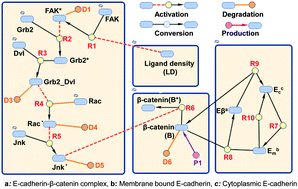
Epithelial to mesenchymal transition (EMT), the process during which epithelial cells lose adhesions with neighbouring cells and get converted to migratory and invasive cells, is closely tied to cancer progression. Cancer progression is also marked by increased deposition and cross linking of fibrillar extracellular matrix (ECM) proteins including collagen and fibronectin, which lead to increase in ECM density and increased cell–matrix adhesions. Thus, an imbalance between cell–matrix and cell–cell adhesions underlies cancer progression. Though several experimental studies have shown a crosstalk between cell–cell and cell–matrix adhesions, the extent to which changes in ECM density can trigger EMT via formation of cell–matrix adhesions and disassembly of cell–cell adhesions remains incompletely understood. In this paper, we have developed a computational framework for studying modulation of cell–cell adhesion by ECM density, integrating findings from multiple studies that connect ECM-mediated adhesion signaling and growth factor signaling with cell–cell adhesion. Here, we have specifically tracked changes in the levels of the E-cadherin–β catenin (Eβ) complex in response to alterations in ECM density. Our results illustrate a tug-of-war between ECM density and E-cadherin in determining Eβ levels both for a single cell as well as for a cell population, with increase in ligand density weakening cell–cell adhesions and increase in E-cadherin levels counterbalancing the effect of ECM density. Consistent with model predictions, lower levels of membrane to cytoplasmic ratios of E-cadherin were observed in MCF-7 human breast cancer cells plated on substrates with increasing collagen density. By performing simulations for a heterogeneous population consisting of both normal and EMT cells, we demonstrate that ligand density and the fraction of EMT cells collectively determine the scattering potential of a cell population. Taken together, our findings are in support of a model where increase in cell–matrix adhesions negatively regulates cell–cell adhesions thereby contributing to EMT and enhanced cellular invasion.

 Please wait while we load your content...
Please wait while we load your content...