UV activation of polymeric high aspect ratio microstructures: ramifications in antibody surface loading for circulating tumor cell selection†
Abstract
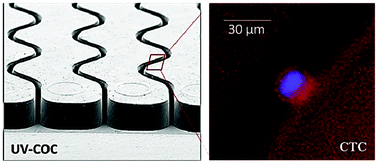
The need to activate thermoplastic surfaces using robust and efficient methods has been driven by the fact that replication techniques can be used to produce microfluidic devices in a high production mode and at low cost, making polymer microfluidics invaluable for in vitro diagnostics, such as circulating tumor cell (CTC) analysis, where device disposability is critical to mitigate artifacts associated with sample carryover. Modifying the surface chemistry of thermoplastic devices through activation techniques can be used to increase the wettability of the surface or to produce functional scaffolds to allow for the covalent attachment of biologics, such as antibodies for CTC recognition. Extensive surface characterization tools were used to investigate UV activation of various surfaces to produce uniform and high surface coverage of functional groups, such as carboxylic acids in microchannels of different aspect ratios. We found that the efficiency of the UV activation process is highly dependent on the microchannel aspect ratio and the identity of the thermoplastic substrate. Colorimetric assays and fluorescence imaging of UV-activated microchannels following EDC/NHS coupling of Cy3-labeled oligonucleotides indicated that UV-activation of a PMMA microchannel with an aspect ratio of ∼3 was significantly less efficient toward the bottom of the channel compared to the upper sections. This effect was a consequence of the bulk polymer's damping of the modifying UV radiation due to absorption artifacts. In contrast, this effect was less pronounced for COC. Moreover, we observed that after thermal fusion bonding of the device's cover plate to the substrate, many of the generated functional groups buried into the bulk rendering them inaccessible. The propensity of this surface reorganization was found to be higher for PMMA compared to COC. As an example of the effects of material and microchannel aspect ratios on device functionality, thermoplastic devices for the selection of CTCs from whole blood were evaluated, which required the immobilization of monoclonal antibodies to channel walls. From our results, we concluded the CTC yield and purity of isolated CTCs were dependent on the substrate material with COC producing the highest clinical yields for CTCs as well as better purities compared to PMMA.
- This article is part of the themed collection: Circulating Tumor Cells

 Please wait while we load your content...
Please wait while we load your content...