Supported molybdenum oxides as effective catalysts for the catalytic fast pyrolysis of lignocellulosic biomass†
Abstract
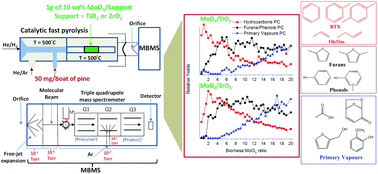
The catalytic fast pyrolysis (CFP) of pine was investigated over 10 wt% MoO3/TiO2 and MoO3/ZrO2 at 500 °C and H2 pressures ≤0.75 bar. The product distributions were monitored in real time using a molecular beam mass spectrometer (MBMS). Both supported MoO3 catalysts show different levels of deoxygenation based on the cumulative biomass to MoO3 mass ratio exposed to the catalytic bed. For biomass to MoO3 mass ratios <1.5, predominantly olefinic and aromatic hydrocarbons are produced with no detectable oxygen-containing species. For ratios ≥1.5, partially deoxygenated species comprised of furans and phenols are observed, with a concomitant decrease of olefinic and aromatic hydrocarbons. For ratios ≥5, primary pyrolysis vapours break through the bed, indicating the onset of catalyst deactivation. Product quantification with a tandem micropyrolyzer–GCMS setup shows that fresh supported MoO3 catalysts convert ca. 27 mol% of the original carbon into hydrocarbons comprised predominantly of aromatics (7 C%), olefins (18 C%) and paraffins (2 C%), comparable to the total hydrocarbon yield obtained with HZSM-5 operated under similar reaction conditions. Post-reaction XPS analysis on supported MoO3/ZrO2 and MoO3/TiO2 catalysts reveal that ca. 50% of Mo surface species exist in their partially reduced forms (i.e., Mo5+ and Mo3+), and that catalyst deactivation is likely associated to coking.


 Please wait while we load your content...
Please wait while we load your content...