Efficient and selective hydrogen peroxide-mediated oxidation of sulfides in batch and segmented and continuous flow using a peroxometalate-based polymer immobilised ionic liquid phase catalyst†
Abstract
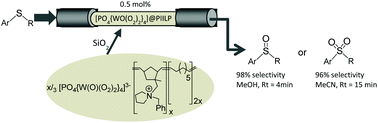
The peroxometalate-based polymer immobilized ionic liquid phase catalyst [PO4{WO(O2)2}4]@PIILP has been prepared by anion exchange of ring opening metathesis-derived pyrrolidinium-decorated norbornene/cyclooctene copolymer and shown to be a remarkably efficient system for the selective oxidation of sulfides under mild conditions. A cartridge packed with a mixture of [PO4{WO(O2)2}4]@PIILP and silica operated as a segmented or continuous flow process and gave good conversions and high selectivity for either sulfoxide (92% in methanol at 96% conversion for a residence time of 4 min) or sulfone (96% in acetonitrile at 96% conversion for a residence time of 15 min). The immobilized catalyst remained active for 8 h under continuous flow operation with a stable activity/selectivity profile that allowed 6.5 g of reactant to be processed (TON = 46 428) while a single catalyst cartridge could be used for the consecutive oxidation of multiple substrates giving activity-selectivity profiles that matched those obtained with fresh catalyst.

 Please wait while we load your content...
Please wait while we load your content...