Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions†
Abstract
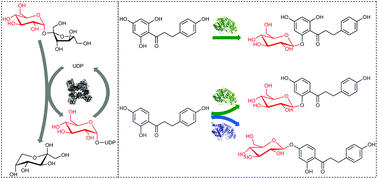
Regioselective O-β-D-glucosylation of flavonoid core structures is used in plants to create diverse natural products. Their prospective application as functional food and pharmaceutical ingredients makes flavonoid glucosides interesting targets for chemical synthesis, but selective instalment of a glucosyl group requires elaborate synthetic procedures. We report glycosyltransferase-catalysed cascade reactions for single-step highly efficient O-β-D-glucosylation of two major dihydrochalcones (phloretin, davidigenin) and demonstrate their use for the preparation of phlorizin (phloretin 2′-O-β-D-glucoside) and two first-time synthesised natural products, davidioside and confusoside, obtained through selective 2′- and 4′-O-β-D-glucosylation of the dihydroxyphenyl moiety in davidigenin, respectively. Parallel biocatalytic cascades were established by coupling uridine 5′-diphosphate (UDP)-glucose dependent synthetic glucosylations catalysed by herein identified dedicated O-glycosyltransferases (OGTs) to UDP dependent conversion of sucrose by sucrose synthase (SuSy; from soybean). The SuSy reaction served not only to regenerate the UDP-glucose donor substrate for OGT (up to 9 times), but also to overcome thermodynamic restrictions on dihydrochalcone β-D-glucoside formation (up to 20% conversion and yield enhancement). Using conditions optimised for overall coupled enzyme activity, target 2′-O- or 4′-O-β-D-glucoside was obtained in ≥88% yield from reactions consisting of 5 mM dihydrochalcone acceptor, 100 mM sucrose, and 0.5 mM UDP. Davidioside and confusoside were isolated and their proposed chemical structures confirmed by NMR. OGT-SuSy cascade transformations present a green chemistry approach for efficient glucosylation in natural products synthesis.

 Please wait while we load your content...
Please wait while we load your content...