Towards the rational biosynthesis of substituted phenazines and phenoxazinones by laccases†
Abstract
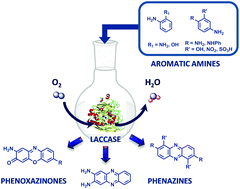
Laccases are multi-copper oxidases that oxidise a wide range of substrates including phenol and aniline derivatives, which could be further involved in coupling reactions leading to the formation of dimeric and trimeric structures. This paper describes the enzyme-mediated dimerisation of several ortho and meta,para-disubstituted aromatic amines into phenazine (“head-to-tail” dimers) and phenoxazinone chromophores. The redox properties of substituted aromatic amines were studied by cyclic voltammetry and the kinetic constants of CotA and Trametes versicolor laccases were measured for selected aromatic amines. The structure of novel enzymatically synthesised phenazine and phenoxazinone dyes using CotA laccase was assessed by NMR and MS. Overall our data show that this enzymatic green process is an efficient alternative to the classic chemical oxidation of aromatic amines and phenols, with an impact on the broad field of applications of these heterocyclic compounds.

 Please wait while we load your content...
Please wait while we load your content...