A solar light-driven, eco-friendly protocol for highly enantioselective synthesis of chiral alcohols via photocatalytic/biocatalytic cascades†
Abstract
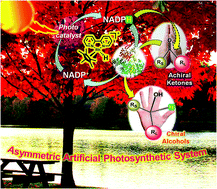
The judicious utilization of solar light for the asymmetric synthesis of optically active compounds by imitating natural photosynthesis introduces a new concept that harnesses this renewable energy in vitro for ultimate transformation into chiral chemical bonds. Herein, we present a comprehensive description of such a biomimetic endeavor towards the design and construction of an asymmetric artificial photosynthesis system that comprises an efficient method of nicotinamide cofactor (NADPH) regeneration under visible light employing a graphene-based light harvesting photocatalyst and its subsequent utilization in an enzyme-catalyzed asymmetric reduction of prochiral ketones to expediently furnish the corresponding chiral secondary alcohols. A detailed optimization study revealed a major dependency of the reaction outcome on the amount of cofactor, photocatalyst and enzyme used, as well as the mode of their addition. A series of structurally diverse ketones bearing an array of (hetero)aryl/alkyl substituents proved to be highly suitable to our photocatalytic–biocatalytic cascade approach, providing (R/S)-1-(hetero)aryl/alkylethanols in excellent enantioselectivities (ee ∼ 95–>99.9%) under mild and environmentally benign conditions. To the best of our knowledge, the synthesis of these enantiopure alcohols employing a visible-light-driven nicotinamide cofactor regeneration strategy has been reported for the first time. Such enantioenriched alcohols act as versatile chiral building blocks for the synthesis of compounds having industrial and pharmaceutical relevance. In addition, this solar-to-chiral chemicals prototype appears advantageous from ecological and economical perspectives. We describe mechanistic pathways to demonstrate how the present catalytic synthesis protocol functions through perfect orchestration between visible-light-driven photocatalysis and biocatalysis to be successively applied in inducing asymmetry in an achiral molecule for the ultimate goal of solar energy utilization in the synthesis of valuable chiral fine chemicals. This work highlights the potential advantages of a bioinspired system to the pertinence of solar energy in asymmetric transformations leading to enantioenriched alcohol precursors, and thus opens up a new field of research that might emerge as an important breakthrough with promising implications towards generating a sustainable and non-fossil/non-nuclear energy future.

 Please wait while we load your content...
Please wait while we load your content...