A recoverable Pd nanocatalyst for selective semi-hydrogenation of alkynes: hydrogenation of benzyl-propargylamines as a challenging model†
Abstract
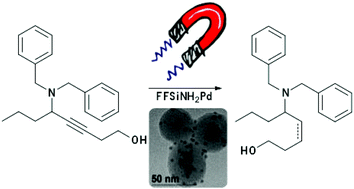
We describe a recyclable heterogeneous palladium nanocatalyst for the selective hydrogenation of alkynes to alkenes. The catalyst was prepared through the decomposition of the organometallic precursor Pd2(dba)3 over a magnetic support, obtaining well-dispersed Pd nanoparticles that formed exclusively on the support surface, with average diameter of 3.5 ± 0.8 nm. The catalytic activity was investigated in the hydrogenation reactions of alkenes and alkynes, and the chemo- and stereoselectivity were evaluated in the hydrogenation of benzyl-propargylamines. The catalyst is highly selective in performing semi-hydrogenation reactions under mild conditions and short reaction times, with good overall yields. Furthermore, it can be easily recovered and recycled, with no leaching of palladium detected, and activities and selectivity retained over multiple reaction cycles.

 Please wait while we load your content...
Please wait while we load your content...