Electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid on supported Au and Pd bimetallic nanoparticles†
Abstract
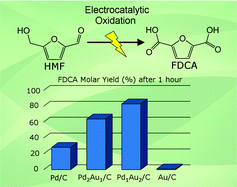
This work explores the potential-dependent electrocatalytic oxidation of 5-hydroxymethylfurfural (HMF) in alkaline media over supported Au and Pd nanoparticles and demonstrates the synergistic effects of bimetallic Pd–Au catalysts for the selective formation of 2,5-furandicarboxylic acid (FDCA). Results from electrolysis product analysis at various electrode potentials, along with cyclic voltammetry of HMF and its oxidation intermediates, revealed the unique catalytic properties of Pd and Au for competitive oxidation of alcohol and aldehyde side-groups present in HMF. Aldehyde oxidation was greatly favored over alcohol oxidation on the Au/C catalyst, which was very active for HMF oxidation to 5-hydroxymethyl-2-furancarboxylic acid (HFCA), however high electrode potentials were required for further oxidation of the alcohol group to FDCA. HMF oxidation on Pd/C followed two competitive routes to FDCA and the pathway was dependent on the electrode potential. Oxidation of aldehyde groups occurred much slower on Pd/C than on Au/C at low potentials, but was greatly enhanced at increased potentials or by alloying with Au. It was found that Pd–Au bimetallic catalysts achieved deeply oxidized products (FFCA and FDCA) at lower potentials than monometallic catalysts and the product distribution was dependent on the electrode potential and surface alloy composition. Bimetallic catalysts with 2 : 1 and 1 : 2 Pd–Au molar ratios (Pd2Au1/C and Pd1Au2/C) exhibited advantages of both single components with facile alcohol and aldehyde group oxidation, resulting in greatly improved HMF conversion rate and selectivity to fully oxidized FDCA.

 Please wait while we load your content...
Please wait while we load your content...