Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids†
Abstract
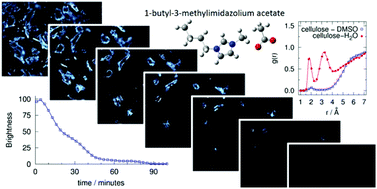
The dissolution of microcrystalline cellulose in 1-butyl-3-methylimidazolium acetate [C4C1Im][OAc] was studied using a solid–liquid equilibrium method based on polarized-light optical microscopy from 30 to 100 °C. We found that [C4C1Im][OAc] could dissolve as much as 25 wt% of cellulose at temperatures below 100 °C. The structure of the composite phase obtained after cooling a solution of 16 wt% of cellulose in [C4C1Im][OAc] was analyzed by low angle X-ray diffraction showing the absence of microcrystalline cellulose, but depicting an extensive long range isotropic ordering. With the aim of improving the dissolution of cellulose in the ionic liquid, dimethyl sulfoxide, DMSO, was added as a co-solvent. It was observed that it enhances the solvent power of the ionic liquid by decreasing the time needed for dissolution, even at low temperatures. In order to understand what makes DMSO a good co-solvent, two approaches were followed. Firstly, we studied experimentally the mass transport properties (viscosity and ionic conductivity) of [C4C1Im][OAc] + DMSO mixtures at different compositions and, secondly, we assessed the molecular structure and interactions around glucose, the structural unit of cellulose, by means of molecular dynamics simulations. As expected, DMSO dramatically decreases the viscosity and increases the conductivity of the mixtures, but without inducing cation–anion dissociation in the ionic liquid. These results were confirmed by molecular simulation as it was found that the presence of a 0.5 mole fraction concentration of DMSO does not significantly affect the hydrogen-bond network in the ionic liquid. Furthermore, molecular dynamics shows that in the [C4C1Im][OAc] + DMSO equimolar mixture, DMSO does not interact specifically with glucose. We conclude that DMSO improves the solvation capabilities of the ionic liquid because it facilitates mass transport by decreasing the solvent viscosity without significantly affecting the specific interactions between cations and anions or between the ionic liquid and the polymer. The behavior of DMSO as a co-solvent was compared with that of water and it was found that water molecules are more probably found near glucose than those of DMSO, thus interfering with ionic liquid–glucose interactions, which might explain the unsuitability of water as a co-solvent for cellulose in ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...