Solvent-free γ-valerolactone hydrogenation to 2-methyltetrahydrofuran catalysed by Ru/C: a reaction network analysis
Abstract
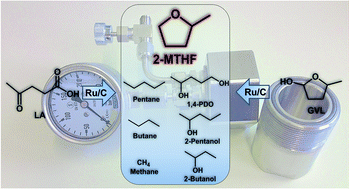
2-Methyltetrahydrofuran (2-MTHF) is considered to be an attractive biomass based platform chemical with high potential as a biofuel compound and as a green solvent. 2-MTHF can be synthesised from bio-based levulinic acid (LA) and γ-valerolactone (GVL). Herein the optimum reaction conditions for the hydrogenation of GVL over Ru/C have been studied. A full conversion of GVL has been obtained under solvent free conditions with a maximum yield of 2-MTHF of 43%. The optimized conditions have been employed in a mechanistic study of the synthesis of 2-MTHF. Several side reactions have been investigated to explore the full reaction network of this heterogeneously catalysed system and to elucidate the factors influencing product selectivity. Additionally an efficient solvent-free hydrogenation reaction of LA into 2-MTHF could be achieved delivering 90% conversion of LA with a yield of 2-MTHF of 61% by removing water from the system in a two-step approach.

 Please wait while we load your content...
Please wait while we load your content...