Controlling C–O, C–C and C–H bond scission for deoxygenation, reforming, and dehydrogenation of ethanol using metal-modified molybdenum carbide surfaces
Abstract
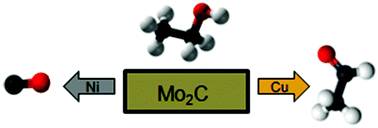
For biomass-derived oxygenate molecules to be fully utilized for chemicals and fuels, control of the bond scission sequence is necessary. Particularly, the C–O, C–H, and C–C bonds must be selectively broken to produce hydrocarbons, aldehydes, and syngas, respectively. Molybdenum carbide (Mo2C) and metal-modified Mo2C may be used to tune the selectivity towards different bond scission pathways. We have investigated how the admetal modification of Mo2C can shift the selectivity towards breaking certain bonds, using ethanol as a probe molecule. Density functional theory (DFT) was used to predict the binding energies of ethanol and reaction intermediates on the Mo2C surfaces. Ultrahigh vacuum (UHV) techniques such as temperature programmed desorption (TPD) and high-resolution electron energy loss spectroscopy (HREELS) were used to verify the activity and reaction pathways on Mo2C and metal-modified Mo2C surfaces. It was seen that the bare Mo2C surface was active for C–O cleavage to produce ethylene. Surface modification with Ni resulted in the preferential C–C bond scission to form syngas, while modification by Cu led to the C–H scission to produce acetaldehyde.
- This article is part of the themed collection: Conversion of biomass with heterogeneous catalysts

 Please wait while we load your content...
Please wait while we load your content...