In vitro transport and satiety of a beta-lactoglobulin dipeptide and beta-casomorphin-7 and its metabolites†
Abstract
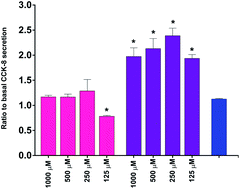
Understanding the digestive behaviour and biological activities of dairy proteins may help to develop model dairy products with targeted health outcomes including increased satiety and healthy weight maintenance. Caseins and whey proteins constitute over 95% of milk proteins with consumption of these proteins associated with increased satiety and a decreased prevalence of metabolic disorders. To investigate the in vitro digestive behaviour and satiety of dairy proteins at the intestinal epithelium, the in vitro transport and hydrolysis of 500–2000 μM β-casomorphin-7 (YPFPGPI or β-CM7) and a β-lactoglobulin (β-Lg) dipeptide (YL) was measured using Caco-2 cell monolayers grown on transwells as a model of the intestinal epithelium. Transport of YL was concentration dependent and ranged from 0.37–5.26 × 10−6 cm s−1, whereas transport of β-CM7 was only detected at 2000 μM and was significantly lower at 0.13 × 10−6 cm s−1. Rapid hydrolysis of β-CM7 in the apical chamber by the Caco-2 cells produced three peptide metabolites: YP, GPI and FPGPI. All of these metabolites were detected in the basolateral chamber after 30 min with both the YP and GPI peptides transporting at a higher rate than intact β-CM7. In vitro satiety was indicated by the secretion of cholecystokinin [26–33] (CCK-8) and glucagon-like peptide 1 (GLP-17–36NH2) in the STC-1 enteroendocrine cell model. CCK-8 secretion was highest in response to β-CM7 followed by the β-CM7 metabolite FPGPI. CCK-8 secretion however was not significantly stimulated by the tri- or dipeptides. Secretion of GLP-1 was not significantly stimulated by β-CM7 or YL. These in vitro results suggest that dairy peptide size enhances CCK-8 secretion, whilst limiting transport across Caco-2 monolayers.
- This article is part of the themed collection: Food Structures, Digestion and Health International Conference

 Please wait while we load your content...
Please wait while we load your content...