Exploring the properties and applications of nanoceria: is there still plenty of room at the bottom?
Abstract
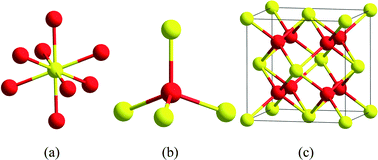
Nanoceria is an exceptionally versatile, commercially valuable catalytic material whose properties vary dramatically from that of the bulk material. Nanoceria's redox properties can be tuned by choice of method of preparation, particle size, nature and level of dopant, particle shape and surface chemistry. The two oxidation states of the cerium element in the lattice make possible the formation of oxygen vacancies which are essential to the high reactivity of the material, its oxygen buffering capability and thus its ability to act as a catalyst for both oxidation and reduction reactions. Ceria has important commercial utility in the areas of chemical mechanical polishing and planarization, catalytic converters and diesel oxidation catalysts, intermediate temperature solid oxide fuel cells and sensors. Its potential future uses include chemical looping combustion, photolytic and thermolytic water splitting for hydrogen production and as a therapeutic agent for the treatment of certain human diseases. We have seen that the method of synthesis, particle size, stabilizing corona, and purity dictate where it is used commercially. Finally, in regards to the prescient words of Dr. Feynman, we note that while there is indeed “plenty of room at the bottom”, there quite possibly exists an optimal nanoceria size of between 2–3 nm that provides maximal reactivity and thermodynamic stability.
- This article is part of the themed collection: Nanoceria Research

 Please wait while we load your content...
Please wait while we load your content...