Chromium(iii) oxidation by biogenic manganese oxides with varying structural ripening†
Abstract
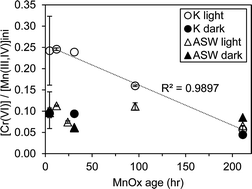
Manganese (Mn) oxides, which are generally considered biogenic in origin within natural systems, are the only oxidants of Cr(III) under typical environmental conditions. Yet the influence of Mn biooxide mineral structural evolution on Cr(III) oxidation under varying geochemical conditions is unknown. In this study we examined the role of light, organic carbon, pH, and the structure of biogenic Mn oxides on Cr(III) oxidation. Aging of Mn oxides produced by a marine bacterium within the widespread Roseobacter clade resulted in structural ripening from a colloidal hexagonal to a particulate triclinic birnessite phase. The structurally diverse Mn oxides were then reacted with aqueous Cr(III) within artificial seawater in the presence or absence of carbon and light. Here we found that Cr(III) oxidation capacity was highest at near neutral pH and in the combined presence of carbon and light. Mn oxide ripening from a hexagonal to a triclinic birnessite phase led to decreased Cr(III) oxidation in the presence of carbon and light, whereas no change in reactivity was observed in the absence of carbon and/or in the dark. As only minimal Cr(III) oxidation was observed in the absence of Mn oxides, these results strongly point to coupled Mn oxide- and photo-induced generation of organic and/or oxygen radicals involved in Cr(III) oxidation. Based on Mn oxide concentration and structural trends, we postulate that Mn(II) produced from the oxidation of Cr(III) by the primary Mn oxide is recycled in the presence of organics and light conditions, (re)generating secondary hexagonal birnessite and thereby allowing for continuous oxidation of Cr(III). In the absence of this Mn oxide regeneration, Cr(III) induced structural ripening of the hexagonal birnessite precludes further Cr(III) oxidation. These results highlight the complexity of reactions involved in Mn oxide mediated Cr(III) oxidation and suggest that photochemical carbon reactions are requisite for sustained Cr(III) oxidation and persistence of reactive Mn oxides.
- This article is part of the themed collection: Geoscience (ESPI)

 Please wait while we load your content...
Please wait while we load your content...