Siderophore-promoted dissolution of chromium from hydroxide minerals†
Abstract
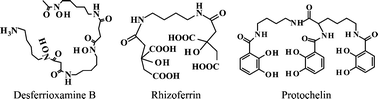
Biomolecules have significant impacts on the fate and transport of contaminant metals in soils and natural waters. Siderophores, Fe(III)-binding agents that are exuded by microbes and plants, may form strong complexes with and promote the dissolution of contaminant metal ions, such as Co(III), U(IV), or Pu(IV). Although aqueous Cr(III)-siderophore complexes have been recognized in the laboratory setting for almost 40 years, few studies have explored interactions of siderophores with Cr-bearing minerals or considered their impacts on environmental chemistry. To better understand the possible effects of siderophores on chromium mobility, we conducted a series of dissolution experiments to quantify the dissolution rates of Cr(III)(OH)3 in the presence of hydroxamate, catecholate, and α-hydroxycarboxylate siderophores over a range of environmentally relevant pH values. At pH = 5, dissolution rates in the presence of siderophores are similar to control experiments, suggesting a predominantly proton-promoted dissolution mechanism. At pH = 8, the sorption of the siderophores desferrioxamine B and rhizoferrin can be modeled by using Langmuir isotherms. The dissolution rates for these siderophores are proportional to the surface concentrations of sorbed siderophore, and extended X-ray absorption fine structure spectra of dissolution products indicates the formation of Cr(III)HDFOB+ and Cr(III)rhizoferrin3− complexes, suggesting a ligand-promoted dissolution mechanism at alkaline pH. Because siderophores promote Cr(III)(OH)3 dissolution at rates similar in magnitude to those of iron hydroxides and the resulting Cr(III)-siderophore complexes may be persistent in solution, siderophores could potentially contribute to the mobilization of Cr in soils and sediments where it is abundant due to geological or anthropogenic sources.

 Please wait while we load your content...
Please wait while we load your content...