Organic matrix effects on the formation of light-absorbing compounds from α-dicarbonyls in aqueous salt solution†
Abstract
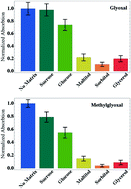
Aqueous-phase reactions of organic compounds are of general importance in environmental systems. Reactions of α-dicarbonyl compounds in the aqueous phase of atmospheric aerosols can impact their climate-relevant physical properties including hygroscopicity and absorption of light. Less-reactive water-soluble organic compounds may contribute an organic matrix component to the aqueous environment, potentially impacting the reaction kinetics. In this work we demonstrate the effects of organic matrices on the self-reactions of glyoxal (Gly) and methylglyoxal (mGly) in aqueous solutions containing ammonium sulfate. At an organic-to-sulfate mass ratio of 2 : 1, carbohydrate-like matrices resembling oxidized organic aerosol material reduce the rate of formation of light-absorbing products by up to an order of magnitude. The greatest decreases in the reaction rates were observed for organic matrices with smaller, more linear molecular structures. Initial UV-Vis spectra, product studies, relative rate data, acidity changes, and viscosity measurements suggest that shifts in carbonyl equilibria, due in part to (hemi)acetal formation with the matrix, reduce the rate of formation of light-absorbing imidazole and oligomer species.
- This article is part of the themed collection: Aquatic photochemistry

 Please wait while we load your content...
Please wait while we load your content...