Degradation of organic pollutants in/on snow and ice by singlet molecular oxygen (1O *2) and an organic triplet excited state†
Abstract
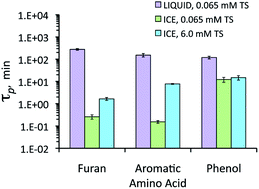
Singlet molecular oxygen (1O*2) can be a significant sink for a variety of electron-rich pollutants in surface waters and atmospheric drops. We recently found that 1O*2 concentrations are enhanced by up to a factor of 104 on illuminated ice compared to in the equivalent liquid solution, suggesting that 1O*2 could be an important oxidant for pollutants in snow. To examine this, here we study the degradation of three model organic pollutants: furfuryl alcohol (to represent furans), tryptophan (for aromatic amino acids), and bisphenol A (for phenols). Each compound was studied in illuminated aqueous solution and ice containing Rose Bengal (RB, a sensitizer for 1O*2) and sodium chloride (to adjust the concentration of total solutes). The RB-mediated loss of each organic compound is enhanced on illuminated ice compared to in solution, by factors of 6400 for furfuryl alcohol, 8300 for tryptophan, and 50 for bisphenol A for ice containing 0.065 mM total solutes. Rates of loss of furfuryl alcohol and tryptophan decrease at a higher total solute concentration, in qualitative agreement with predictions from freezing-point depression. In contrast, the loss of bisphenol A on ice is independent of total solute concentration. Relative to liquid tests, the enhanced loss of tryptophan on ice during control experiments made with deoxygenated solutions and solutions in D2O show that the triplet excited state of Rose Bengal may also contribute to loss of pollutants on ice.
- This article is part of the themed collection: Aquatic photochemistry

 Please wait while we load your content...
Please wait while we load your content...