On rechargeability and reaction kinetics of sodium–air batteries†
Abstract
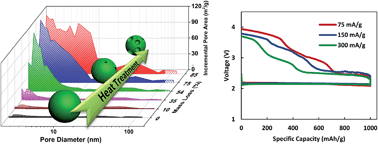
Rechargeable metal–air batteries are widely considered as the next generation high energy density electrochemical storage devices. The performance and rechargeability of these metal–air cells are highly dependent on the positive electrode material, where oxygen reduction and evolution reactions take place. Here, for the first time, we provide a detailed account of the kinetics and rechargeability of sodium–air batteries through a series of carefully designed tests on a treated commercial carbon material. Surface area and porous structure of the positive electrode material were controlled in order to gain detailed information about the reaction kinetics of sodium–air batteries. The results indicate that discharge capacity is linearly correlated with surface area while morphology of the solid discharge product is strongly dependent on specific surface area and pore size. Furthermore, it was found that the chemical composition of discharge products as well as charging overpotential is affected by discharge reaction rate.


 Please wait while we load your content...
Please wait while we load your content...