A reversible Br2/Br− redox couple in the aqueous phase as a high-performance catholyte for alkali-ion batteries†
Abstract
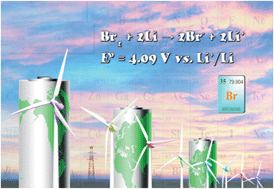
An aqueous catholyte for Li-ion redox batteries employing reversible Br2/Br− redox reactions is investigated. The catholyte uses lithium bromide as the active material and potassium bromide aqueous solution as the supporting electrolyte. By tuning the acidity of the catholyte, stable cyclability of the cell has been achieved at room temperature with a discharge potential of ∼3.9 V, a reversible capacity of 290 mA h g−1, and a specific power density of ∼1000 W kg−1. Compared with the catholytes used in conventional redox-flow batteries, the reported catholyte is heavy-metal-free and can be operated in moderate acidity, providing an alternative option for sustainable electrical energy storage.

 Please wait while we load your content...
Please wait while we load your content...