‘Dry bases’: carbon dioxide capture using alkaline dry water†
Abstract
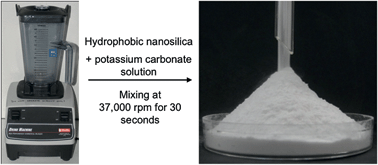
An alkaline form of ‘dry water’—a ‘dry base’—is prepared by the high-speed mixing of aqueous solutions of metal carbonates or organic amines with hydrophobic silica nanoparticles. Despite being mostly water, the dry base looks and flows like a powder, and adsorbs CO2 rapidly without any mixing because of its high surface-to-volume ratio. Unlike normal aqueous base solutions, dry bases can be non-corrosive because they do not readily wet surfaces.

 Please wait while we load your content...
Please wait while we load your content...