Substituent effects and chemoselectivity of the intramolecular Buchner reaction of diazoacetamide derivatives catalyzed by the di-Rh(ii)-complex†
Abstract
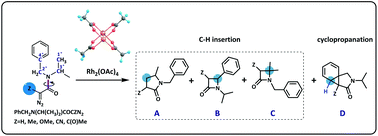
A density functional theory (DFT) study was performed to reveal that the substituent effects in the α-site have an effect on the chemoselectivity of the intramolecular Buchner reaction of diazoacetamide catalyzed by Rh2(OAc)4. The substituent effect is investigated considering five different groups (Z = –Me, –OMe, –H, –CN and –C(O)Me) in the substrates. The substituent group in the α-site changes the electronegativity of the C-atom in carbene and affects the chemoselectivity. The basis of chemoselectivity is the distribution of products that was analyzed by DFT calculations. The barrier energy of the favorable pathway is clearly lower than that of the other pathways. Nucleophilic substituent groups, such as –H, –OMe and –Me, are regarded as electron-donating groups, which increase the electropositivity of the C-atom in carbene compounds and improve the reactivity of the aromatic addition reaction. Electrophilic substituent groups, such as –CN and –C(O)Me, are regarded as electron-withdrawing groups, which decrease the electropositivity of the C-atom in carbene compounds and favor the C–H activation step. The computational results showed that the main product is cycloheptatriene when Z = –H/–OMe. The main product is β-lactam when the substituent group is –CN/–C(O)Me. When the substituent group is –Me, the products are a mixture of γ-lactams, β-lactams and cycloheptatriene.

 Please wait while we load your content...
Please wait while we load your content...