Spectroelectrochemical identification of charge-transfer excited states in transition metal-based polypyridyl complexes
Abstract
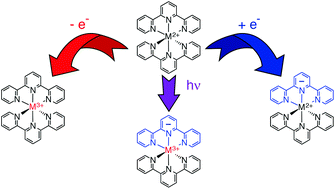
Identification of transient species is a necessary part of delineating the kinetics and mechanisms associated with chemical dynamics; when dealing with photo-induced processes, this can be an exceptionally challenging task due to the fact that spectra associated with excited state(s) sampled over the course of a photochemical event often cannot be uniquely identified nor readily calculated. Using Group 8 complexes of the general form [M(terpy)2]2+ and [M(bpy)3]2+ as a platform (where terpy is 2,2′:6′,2′′-terpyridine and bpy is 2,2′-bipyridine), we demonstrate how spectroelectrochemical measurements can serve as an effective tool for identifying spectroscopic signatures of charge-transfer excited states of transition metal-based chromophores. Formulating the metal-to-ligand charge-transfer (MLCT) excited state(s) as M3+–L−, the extent to which a linear combination of the spectra of the oxidized and reduced forms of the parent complexes can be used to simulate the characteristic absorptions of MLCT-based transient species is examined. Quantitative agreement is determined to be essentially unachievable due to the fact that certain transitions associated with the optically prepared excited states are either overcompensated for in the spectroelectrochemical data, or simply cannot be replicated through electrochemical means. Despite this limitation, it is shown through several illustrative examples that this approach can still be extremely useful as a qualitative if not semi-quantitative guide for interpreting time-resolved electronic absorption data of charge-transfer compounds, particularly in the ultrafast time domain.
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...