Gold(i) thiolates containing amino acid moieties. Cytotoxicity and structure–activity relationship studies
Abstract
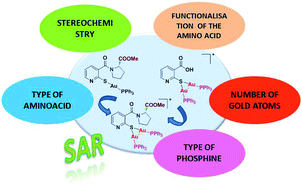
Several gold(I) complexes containing a thiolate ligand functionalised with several amino acid or peptide moieties of the type [Au(SPyCOR)(PPh2R′)] (where R = OH, amino acid or dipeptide and R′ = Ph or Py) were prepared. These thiolate gold complexes bearing biological molecules possess potential use as antitumor agents. Cytotoxicity assays in different tumour cell lines such as A549 (lung carcinoma), Jurkat (T-cell leukaemia) and MiaPaca2 (pancreatic carcinoma) revealed that the complexes exhibit good antiproliferative activity, with IC50 values in the low micromolar range. Several structural modifications such as in the type of phosphine, number of metal atoms and amino acid (type, stereochemistry and functionalisation) were carried out in order to establish the structure–activity relationship in this family of complexes, which has led to the design of new and more potent cytotoxic complexes. Observations of different cellular events after addition of the complexes indicated the possible mechanism of action or the biological targets of this type of new gold(I) drug.

 Please wait while we load your content...
Please wait while we load your content...