Towards a better understanding of magnetic exchange mediated by hydrogen bonds in Mn(iii)/Fe(iii) salen-type supramolecular dimers†
Abstract
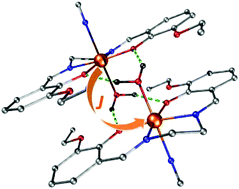
A thorough study of structural and magnetic properties was performed on a series of trinuclear and dinuclear Mn(III)/Fe(III) complexes consisting of [M(L4)(Solv)]+ and [Fe(CN)5(NO)]2− moieties (M = Fe(III) or Mn(III), Solv = H2O or CH3OH, L4 = tetradentate salen-type ligands), in which dominant magnetic exchange is mediated by OS–H⋯OPh hydrogen bonds in [M(L4)(Solv)]+⋯[M(L4)(Solv)]+ supramolecular dimers. As deduced from magnetic analysis involving the determination of zero-field splitting (ZFS) parameters for Mn(III) and Fe(III) ions, as well as from comprehensive DFT calculations and modelling, it may be concluded that the strength of the magnetic exchange is correlated with the number of hydrogen bonds and with the OPh⋯OS distance between the phenolic oxygen atom of the salen-type ligand (OPh) and the oxygen atom of the solvent molecule coordinated to the adjacent metal atom (OS).


 Please wait while we load your content...
Please wait while we load your content...