Co-molten solvothermal method for synthesizing chalcopyrite CuFe1−xCrxS2 (x ≤ 0.4): high photocatalytic activity for the reduction of nitrate ions
Abstract
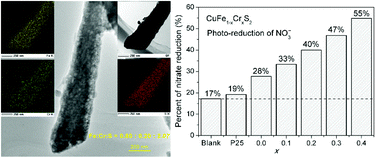
In literature, it is very difficult to obtain sulfides with Cr3+ in tetrahedral coordination. Here, a thiourea–oxalic acid co-molten solvothermal method was applied to synthesize chalcopyrite CuFe1−xCrxS2 (x ≤ 0.4) solid solutions. We propose that oxalic acid plays an important role in the crystallization of CuFe1−xCrxS2 and can considerably restrain the formation of other undesirable impurities. The successful incorporation of Cr3+ was confirmed by powder XRD, SEM and EDX mapping (2D elemental distribution). The UV-Vis reflectance spectra of CuFe1−xCrxS2 suggest that the bandgap energies decrease from 0.80 to 0.61 eV along with an increase in the Cr3+ concentration. All the CuFe1−xCrxS2 (0 ≤ x ≤ 0.4) samples show considerably higher photocatalytic activities than P25 toward the reduction of nitrate ions in aqueous solution. We speculate that the thiourea–oxalic acid co-molten method may not only be effective to synthesize Fe3+–Cr3+ sulfides, but can also be helpful to incorporate Cr3+ to other sulfide systems with MS4 tetrahedra.

 Please wait while we load your content...
Please wait while we load your content...