Tuning of the dimensional linkage from the complex to the framework by thermal conversion in the system Fe/Cl/piperazine†
Abstract
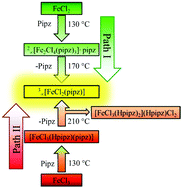
An attempt to control the three structural features linkage, structural dimensionality and coordination mode is presented for the combination of FeII and FeIII chlorides together with the ditopic ligand piperazine (pipz). Thermal conversion of small units like complexes into highly aggregated structures such as coordination polymers is demonstrated for the framework formation of 3∞[FeIICl2(pipz)] starting from both FeCl2 and FeCl3. Depending on the oxidation state of iron, different reaction paths and metastable products are observed. Iron(II)chloride reacts with piperazine to form the previously unknown 2-dimensional network, 2∞[FeII2Cl4(pipz)3]·pipz, which can be thermally converted into the 3-dimensional framework at elevated temperatures by release of piperazine. The use of iron(III)chloride starts with an internal redox reaction giving the divalent complex [FeIICl3(Hpipz)(pipz)] in the first step, followed by thermal conversion yielding the framework 3∞[FeIICl2(pipz)] and the salt [FeIICl3(Hpipz)(pipz)](Hpipz)Cl2. Both thermal conversion mechanisms were further investigated by in situ IR-spectroscopy, differential thermal analysis and thermogravimetry.

 Please wait while we load your content...
Please wait while we load your content...