Spin–orbit effects in square-planar Pt(ii) complexes with bidentate and terdentate ligands: theoretical absorption/emission spectroscopy†
Abstract
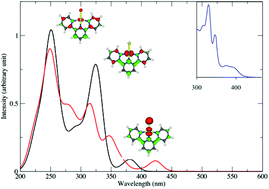
The absorption and emission properties of five Pt(II) planar complexes with bidentate ligands, namely [Pt(bpy)Cl2] (bpy = 2,2′-bipyridine) 1 and [Pt(ppy)Cl2]− (ppy = 2-phenylpyridine) 2, and terdentate ligands, namely [Pt(tpy)Cl]+ (tpy = 2,2′:6′,2′′-terpyridine) 3, [Pt(phbpyR)Cl] (phbpy = 6-phenyl-2,2′-bipyridine; R = H) 4 and [Pt(dpybR)Cl] (dpyb = 2,6-di(2-pyridyl)benzene; R = CH3) 5 were investigated by means of density functional theory (DFT) and time-dependent DFT (TD-DFT) methods including solvent correction and spin–orbit coupling (SOC). The DFT optimized structures of the five complexes in the electronic ground state are in agreement with the experimental X-ray data and the theoretical absorption spectra reproduce quantitatively the main features of the experimental spectra. It is shown that the structures remain nearly planar in the low-lying singlet and triplet excited states of charge transfer character, metal-to-ligand (MLCT) or halide (Cl) to ligand (XLCT) whereas a significant distortion corresponding to the out-of-plane-bending of the Pt–Cl bond characterizes the geometry of the metal-centered (MC) states. In cyclometalated complexes 2, 4 and 5 this distortion is energetically unfavorable and not competitive with radiative decay via the low-lying MLCT and XLCT excited states. The absorption spectra of all complexes are significantly affected by spin–orbit coupling (red-shift and broadening), especially in the non-cyclometalated complexes 1 and 3 characterized by the presence of pure low-lying 3MC states. The SOC effects are less important in the terpyridine complex 3 the lowest part of its spectrum being contaminated by mixed 3MLCT/3XLCT states. In the cyclometalated complexes 4 and 5 the presence of several LC states in the lowest part of the spectra is responsible for a small shift to the red as compared to the other complexes. The solvatochromism that characterizes the absorption of this class of molecules in the visible region is interpreted by the MLCT/XLCT mixed character of the excited states in this energy domain (400–450 nm). Indeed the solvent dependent XLCT contribution will control the magnitude of SOC in these excited states and will move the band to the red region when diminishing and to the blue region when increasing. As far as emission is concerned it is shown that strongly distorted non-radiative MC state's minima, situated below the charge transfer state's minima (ΔE = −0.3 eV to −0.8 eV) are easily accessible upon irradiation in the visible region in complexes 1 and 3, and in 2 to a lesser extent, leading to no or low luminescence at room temperature. In contrast, the minima of the emissive states of mixed MLCT/XLCT/LC character are efficiently populated in 4 and 5. The luminescence of complex 5, cyclometalated in the axial position, is particularly efficient because the minimum of the lowest emissive state is well separated from those of the MC states (ΔE = +0.23 eV) in contrast to its analog, complex 4, cyclometalated in the lateral position where the emissive MLCT/LC state minimum is nearly degenerate with the lowest MC state minimum (ΔE = +0.01 eV).
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...